Dian L. Baker, Karen K. Giuliano, Chantal Worzala, Annie Cloke, Lu Zawistowich
Hospital-acquired pneumonia (HAP), which includes both nonventilator-associated pneumonia (NVHAP) and ventilator-associated pneumonia (VAP), is one of the most common health care-associated infections (HAIs), constituting approximately 25 percent of HAIs in acute care hospitals. Clinical guidelines and several major health systems’ initiatives indicate that this widespread and costly condition can largely be prevented.
In light of the growing evidence that HAP continues to negatively impact patient outcomes, policy makers (including the Centers for Medicare and Medicaid Services [CMS]) should reinvigorate their focus on this priority problem. To date, no acute inpatient hospital quality program implemented by Medicare includes measures to prevent this condition, although CMS first considered addressing HAP through its inpatient hospital quality programs in 2008 and has started to focus on it in post-acute settings (Note 1). In this article, we:
- Review the evidence that supports the prevention of HAP as a priority for patient safety,
- Review clinical guidelines and health systems’ efforts and successes aimed at HAP prevention, and
- Outline the importance of policy makers’ actions on HAP prevention to improve patient safety and outcomes and potentially reduce costs, both overall and for the Medicare program.
Hospital-Acquired Pneumonia Is Common And Costly
In September 2021, the Joint Commission issued a new QuickSafety advisory—“Preventing nonventilator hospital-acquired pneumonia”—which identifies NVHAP as a significant patient safety and quality issue and includes recommended actions for NVHAP prevention. This step follows the National Organization to Prevent Hospital-Acquired Pneumonia (NOHAP), a group of prominent US health care leaders, issuing a call to action to address NVHAP. According to the Joint Commission, “one in every 100 hospitalized patients will be affected by NVHAP.” VAP is the most commonly reported HAI among patients receiving mechanical ventilation. Furthermore, research indicates that the most common infection leading to sepsis—a potentially life-threatening condition where the body’s response to an infection damages its own tissues—is pneumonia. One study estimated that 36.3 percent of patients with NVHAP developed sepsis and cites research estimating that 50 percent of sepsis cases are associated with pneumonia.
Research has also found that HAP (both NVHAP and VAP) is associated with longer hospital length-of-stay, higher overall health care costs, and increased morbidity and mortality. A 2018 analysis found that NVHAP had a mortality rate of 13 percent and, based on analysis of previous studies, contributed to longer hospital length-of-stay (13–28 days) and had associated acute care costs ranging from $28,000 to $40,000. Another analysis of previous research found that VAP has high rates of mortality (up to 17 percent), increases hospital stay (by 6 to 25 days) and leads to significant costs to the system ($12,000 to $40,000 per episode).
Together, this research demonstrates that HAP is a high-volume, high-cost HAI, making it a crucial target for CMS to include in inpatient quality programs.
COVID-19 Highlights The Need To Focus On Patient Safety And Infection Control
In addition to the growing body of research on the effects of HAP, early data on COVID-19’s impacts on HAIs underscores the need for CMS to prioritize patient safety and infection control, which must include a strong focus on HAP prevention. During the COVID-19 pandemic, hospitals faced unprecedented challenges in caring for patients infected by the virus, protecting health care workers, and helping to lead vaccination efforts in their communities. The response and management of COVID-19 cases quickly became the main focus of infection prevention efforts, decreasing the time available to surveil and prevent the more traditional HAIs. Consistent with early studies, CMS and the Centers for Disease Control and Prevention (CDC) reported that the rate of central-line-associated bloodstream infections, catheter-associated urinary tract infections, ventilator-associated events (VAE)—which includes VAP—and methicillin-resistant Staphylococcus aureus bacterium infections had increased in US hospitals since the beginning of the pandemic. This points to the need to continue to focus on preventable HAIs and to include HAP.
Policy Makers Have Yet To Include HAP In Acute Inpatient Hospital Prevention Efforts
Congress and CMS have acted to reduce rates of certain hospital-acquired infections through the Hospital-Acquired Conditions (HACs) and Present on Admission (POA) policy and the Hospital-Acquired Condition Reduction Program (HACRP), which address HAIs that are preventable and high volume and/or high cost. Yet, HAP has not been included in these programs.
CMS first considered adding a measure on VAP to the HAC POA program in the FY 2008 Inpatient Prospective Payment Systems proposed rule, but the agency did not finalize this proposal, citing concerns about coding, diagnosability, and preventability. In subsequent regulatory decision making on HAIs, CMS included catheter-associate urinary tract infections, Methicillin-resistant Staphylococcus aureus (MRSA), and select surgical site infections in the HACRP, despite a significantly lower volume of many of these HAIs as compared to HAP. In adding MRSA, CMS specifically noted that HAIs “must be reasonably—and not completely—preventable for inclusion in the HACRP.” These conditions offer examples of how addressing HAP through acute inpatient hospital quality programs would be consistent with the agency’s previous efforts and could incentivize actions to reduce this prevalent, costly, and largely preventable complication.
Policy makers have taken some steps to address VAP in other settings or programs, but only on a short-term or limited basis.
- In 2011, CMS and the Center for Medicare and Medicaid Innovation’s Partnership for Patients Hospital Engagement Network led a collaborative national patient safety initiative to address a broad range of hospital-acquired conditions, including VAEs such as VAP (Note 2). The Partnership for Patients has since ended.
- In FY 2015, CMS included a VAE measure in the Long-Term Care Hospital quality reporting program. However, the measure was removed in FY 2019 as part of CMS’s Meaningful Measures Initiative.
- More recently, CMS’s FY 2022 Skilled Nursing Facility Prospective Payment System Final Rule added a measure on health care-associated infections requiring hospitalization, which includes pneumonia, to the skilled nursing facility quality program.
Most recently, CMS and the CDC highlighted the need to focus on patient safety as a key part of building a resilient health care system in response to increased rates of health care-associated infections during the COVID-19 pandemic. HAP prevention must be included as a key part of this effort. Including HAP is consistent with CMS’s inclusion of VAEs as a priority area and measure need for the HACRP in 2019, 2020, and 2021. Despite prioritizing VAEs, however, CMS has not proposed any new policy to address HAP in the inpatient setting.
Clinical Guidelines And Health System Initiatives Show HAP May Be Prevented
While stakeholders have raised concerns about the ability to diagnose and prevent HAP, the recent proliferation of guidelines for HAP prevention, coupled with successful reductions in HAP at major health systems, demonstrates that both issues can be addressed. In addition, research is underway to allow for easy and efficient electronic health record data extraction to identify NVHAP.
Clinical guidelines and recommendations for the prevention of HAP date back to 2003 and have been issued by several federal agencies, including the CDC, the Agency for Healthcare Research and Quality (AHRQ), and the Department of Veterans Affairs (VA). Professional health care and infectious disease societies have also issued similar guidelines, indicating that implementation of HAP programs can reduce harm and improve patient outcomes (see exhibit 1).
Exhibit 1: Timeline of HAP prevention guidelines
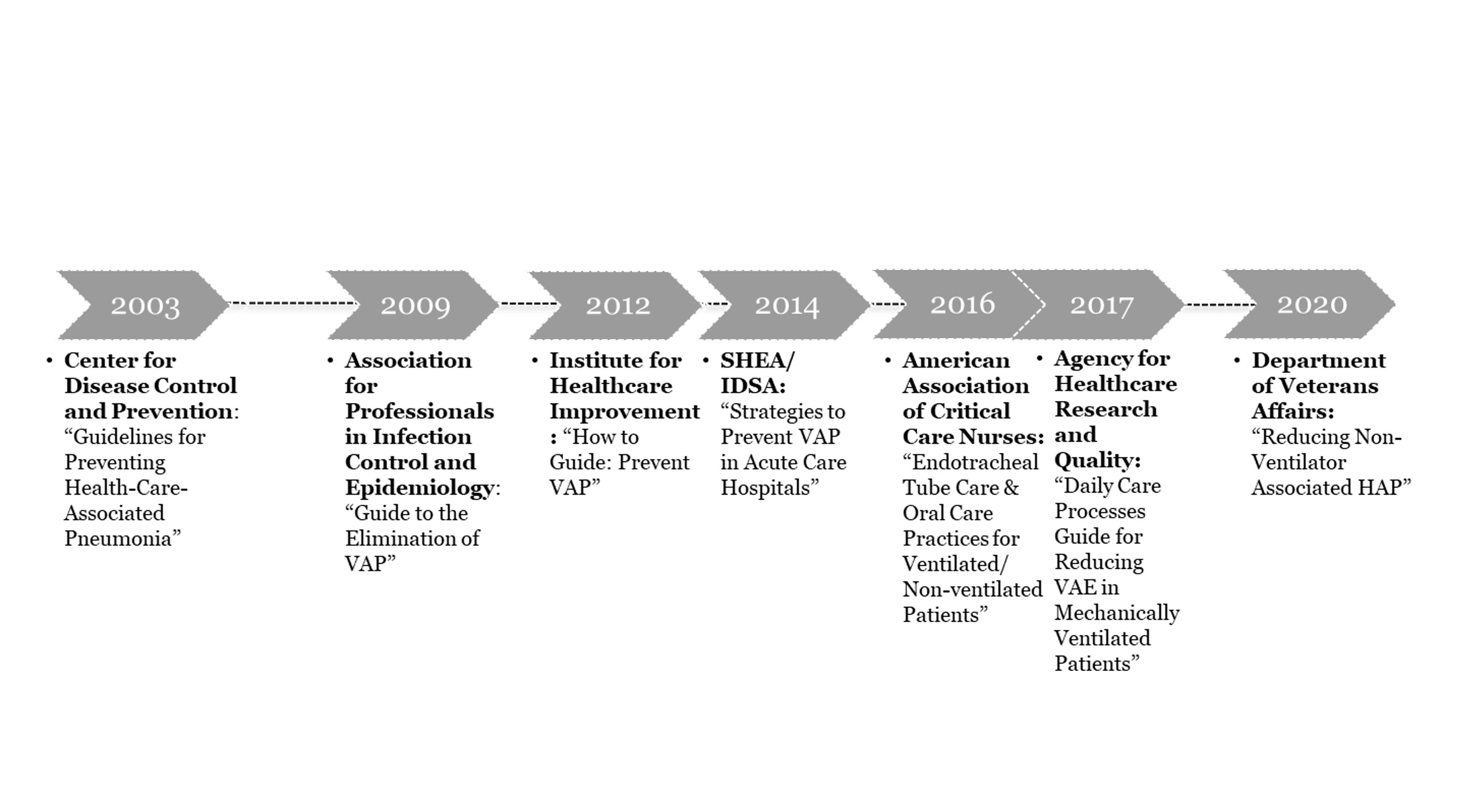
Sources: CapView Strategies Analysis. (Links to the additional guidelines in this exhibit are listed in Note 3.)
In addition to clinical guidelines, several health system initiatives have shown promising results—especially in preventing NVHAP. Studies examining NVHAP prevention efforts in integrated health systems such as Kaiser Permanent Northern California, Sutter Health, and the Veterans Health Administration (VHA) show that systemwide initiatives can effectively reduce the incidence of NVHAP and underscore that prevention efforts can work. Kaiser Permanente Northern California implemented an initiative to prevent NVHAP for high-risk patients and decreased NVHAP rates from 5.9 per 1,000 admissions to 1.8 per 1,000 admissions, or 69 percent, between 2012 and 2018. The same study also found a 73 percent reduction in NVHAP mortality rates (1.1 to 0.3 per 1,000 admission) and a reduction in broad-spectrum antibiotic use after the intervention was implemented. Sutter Health’s NVHAP prevention initiative led to a significant reduction in the incidence of NVHAP, with results sustained over four years. A study found that Sutter Health’s protocol, of a daily oral care regimen, led to a 23–46 percent reduction in the incidence of NVHAP between 2013 and 2016. A VHA initiative known as HAPPEN, which aimed to reduce incidence of NVHAP by implementing an oral care regime, started in one facility in 2016 and has been expanded to all VA hospitals nationally.
These efforts show that systems that have applied a consistent diagnostic method and prevention protocol have successfully reduced their incidence of NVHAP and provide templates that can be replicated within other hospital systems. As such, policy makers should not allow the lack of a gold standard for diagnosing HAP to impede their needed action.
Policy Maker Action Is Needed To Address HAP, Improve Medicare Beneficiary Outcomes, And Reduce Program Costs
As emphasized recently by CMS and the CDC, prioritizing patient safety and infection control will be critical components of any strategy to rebuild and strengthen public health after COVID-19. As evidence demonstrating the benefits of addressing HAP—with a significant focus on NVHAP—grows, stakeholder calls to focus on HAP prevention have also increased, most notably through the NOHAP call to action.
Given the growing body of research and evidence demonstrating the prevalence and impacts of pneumonia, Congress and CMS should reinvigorate their focus on this priority problem and take action on HAP prevention, either within existing quality programs or through innovative efforts. A number of policy levers are available to address this serious gap in patient safety. In the near term, policy makers could incorporate an existing HAP-related measure into Medicare hospital or other quality programs—or into other reforms to programs promoting quality and safety in Medicare. Policy makers also could facilitate collaborative efforts to build on and increase adoption of the successful models demonstrated through initiatives at the VHA and other health systems. In the long term, there may be opportunities to develop more accurate quality measures for the prevention of HAP, or to use the Quality Payment Program or the Center for Medicare and Medicaid Innovation’s authority to improve quality of care and patient safety.
Among the many lessons learned from the COVID-19 pandemic is the need to reprioritize infection control within the health system. Growing evidence demonstrates that preventing HAP is an imperative step toward accomplishing this goal, and that HAP must be addressed by policy makers to protect patient safety and strengthen the health system for the future.
Note 1
Measures that address the treatment of individuals being treated for pneumonia as their primary condition have been used, but no measures to incentivize the prevention of pneumonia acquired while in the hospital after being admitted for another condition have been incorporated into hospital quality programs.
Note 2
The interim evaluation of Partnership for Patients conducted in 2015 found that national rates of inpatient harm, including VAE/VAP, markedly improved during the time the model was operating, but did not find differences in the rate of improvement across hospitals that did and did not participate. However, the evaluation found that certain project activities, such as peer-to-peer networking, skills training, and virtual consultation or coaching, were “associated with a greater likelihood of a hospital implementing operational changes targeting harm reduction compared to those that did not participate in these activities.” In addition, the AHRQ found overall reductions in HAIs from 2014 to 2017.
Note 3
- American Association of Critical Care Nurses: “Endotracheal Tube Care & Oral Care Practices for Ventilated/ Non-ventilated Patients”
- Association for Professionals in Infection Control and Epidemiology: “Guide to the Elimination of VAP”
- Institute for Healthcare Improvement: “How to Guide: Prevent VAP”
- SHEA/ IDSA: “Strategies to Prevent VAP in Acute Care Hospitals”
Authors’ Note
Stryker provided funding to support the research and analysis of this project.
